Reversible changes are changes that can be undone or reversed. Melting, freezing, boiling, evaporating, condensing, dissolving and also, changing the shape of a substance are examples of reversible changes.
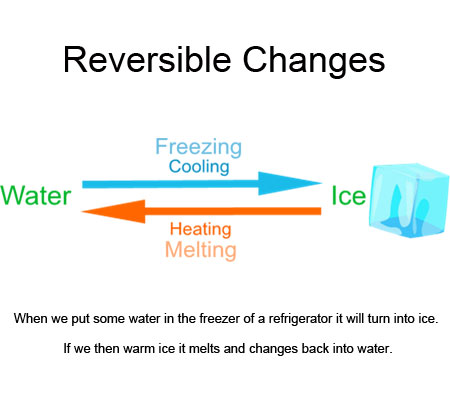
Reversible changes are changes that can be undone or reversed.
Physical changes
Reversible changes usually change the physical appearance of a substance. Therefore, reversible changes are also called physical changes.
E.g. – Water can change into ice. Ice can change into water. Here, only the state of the substance liquid water changes, but not the substance water.
Reversible changes fall under different processes. They are;
(Boiling and evaporation are the two types of vaporization)
Now let’s see the examples of reversible changes or physical changes involved in these different processes.
Following are some of the reversible changes examples.
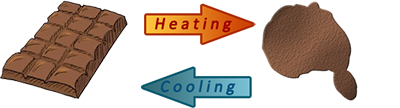
Example (1) – Melting chocolate
When chocolate is heated until it melts, the melted chocolate can be changed back into solid chocolate by cooling or freezing.


Example (2) – Melting candle wax
When candle wax is heated, the solid wax melts and becomes a liquid. If you cool the molten wax, it becomes a solid again.

Example (3) – Melting ice cubes
When you keep some cubes of ice outside the freezer for a few minutes, you can see how they melt and turn into liquid water.
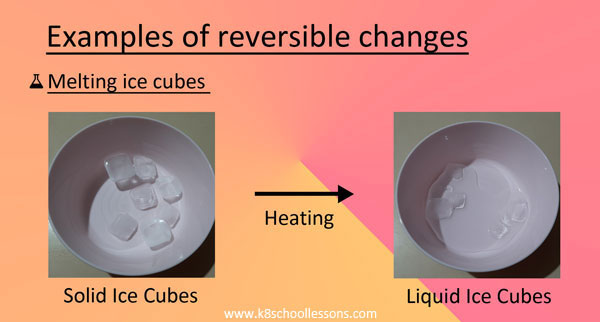
If you pour this water in a tray of ice cubes and keep in the freezer for some time, you can make ice cubes back.
Example (4) – Melting butter
Heat some butter and see how it melts. Keep the melted butter back in the fridge and get solid butter back.


Example (5) – Melting metal
People cast metal into shapes by melting them into a liquid. Melted metal is poured into molds. As the metal cools, it solidifies. This is how people make metal objects with different shapes. Alluminium, iron, lead, copper, gold and silver are popular types of metal that are melted. Also, brass, bronze and steel are some examples of metal alloys created by melting metal.

Example (6) – Melting plastic
Plastic can also be melted and pour into different molds to get different shapes. After cooling down, you can get solid plastic back.
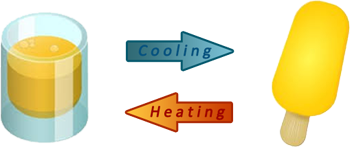
Example (1) – Making ice lollies
When orange juice is frozen to make ice lollies, the ice lollies can be changed back into liquid orange juice by heating.

Example (2) – Making ice cubes
When we put some water in the freezer of a refrigerator it will turn into ice. If we then warm ice it melts and changes back into water.


Example (3) – Making jelly
You can freeze a mixture of liquid jelly and turn it into solid jelly. If you keep solid jelly outside the fridge for sometime you can get liquid jelly back.

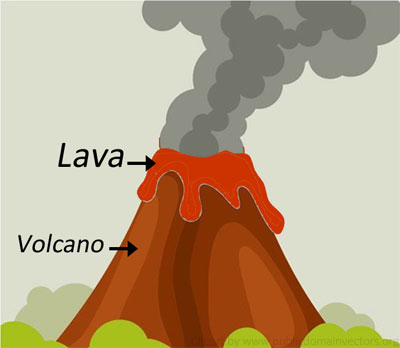
Example (4) – Freezing lava
Lava is molten rock. Rock can be melted due to the extreme heat at the centre of the earth and turns into a liquid called ‘magma’. Magma gushes out of the earth’s weakest surfaces as a volcanic eruption. The runny magma that flows from the volcano is called ‘lava’. Lava that runs out of volcanoes cools down quickly.
You can boil some water and turn it into a gas, which is water vapour. We also call this ‘steam’. This process is called ‘evaporation’.
If you could capture all the steam that is made when a kettle boils, you could turn it back to water by letting it cool. This process is called ‘condensation’

Substances like iodine and dry ice can directly change to the gaseous state when they are heated. This process is called ‘sublimation’.
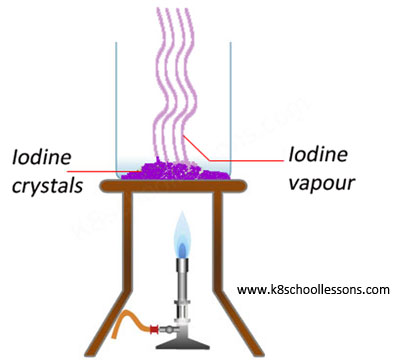
Example (1) – Heating iodine crystals
When solid iodine crystals are heated they directly form a purple gas without passing through a liquid state.
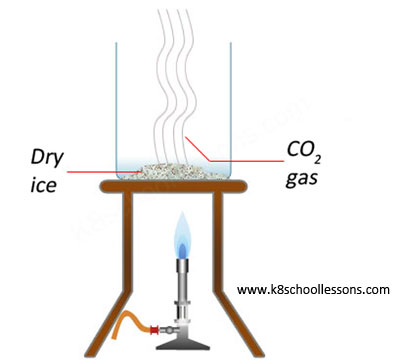
Example (2) – Heating dry ice
Dry ice is carbon dioxide in its solid form. It turns directly into a gas when heated, instead of melting into a liquid.
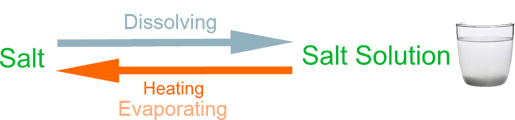
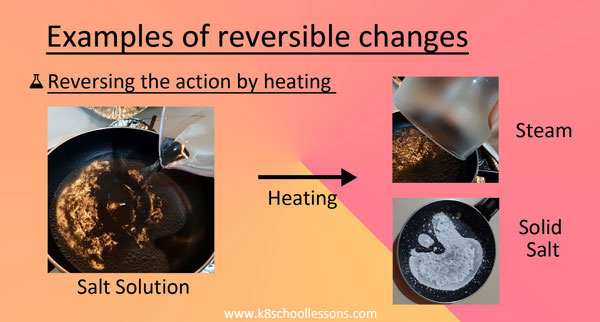
Example (1) – Salt solution
When salt is mixed with water it disappears because it dissolves in the water to make salty water. We call this salt solution. The salt in the solution can be recovered by boiling off the water.
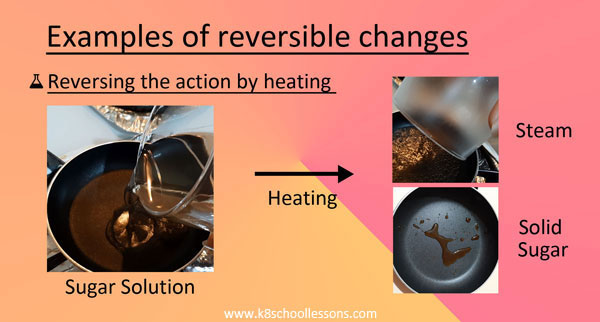
Example (2) – Sugar solution
You can make a sugar solution by dissolving sugar crystals in water. When you heat the solution, the water in it will evaporate leaving behind the sugar.
We know that physical changes are reversible. But not all of them. Some physical changes occurred by changing the shape of a substance are not reversible. Let’s see which are reversible and which are not.
Example (1) – Blowing up a balloon
When you blow up a balloon the shape of the balloon is changed. If you let the air inside the balloon go it will come back to its usual shape. This physical change is reversible.

Example (2) – Crumpling a piece of paper
When you crumple a piece of paper it will become creased and wrinkled. However, neither the amount of matter in the paper nor the material the paper is made is changed. This change is reversible.
Example (3) – Cutting, tearing and breaking substances
When you cut a piece of wood in half, you change its shape. But you do not alter the way the wood is made.
Similarly, when you cut, tear or break something you just alter its shape by dividing the substance into some parts. The amount of matter in the substance and the material which the substance is made are not changed.
These types of changes are physical changes which are not reversible.

Example (4) – Molding clay
When you mold some clay into a pot, the shape of clay is changed, but no new matter or material is formed. You can turn the pot back into the same chunk of clay before you bake it in the kiln. This physical change is reversible.

Example (5) – Sharpening a pencil
When you sharpen a pencil, you change the physical appearance or the shape of the pencil. Also, some amount of matter is separated from the pencil, but no matter or material of the pencil is chemically changed. This physical change is not reversible.

Example (6) – Stretching a rubber band
When you stretch a rubber band, you alter its shape, but it is still made of rubber. When you let it go it will come back to its usual shape. This physical change is reversible.
Example (7) – Squashing a sponge
Similarly, when you squash a sponge, you alter its shape, but it is still made of sponge. When you let it go it will come back to its usual shape. This physical change is also reversible.

Example (1) – Sand and gravel mixture
A sand and gravel mixture consists of solid particles of different sizes. Sieving is the best method to separate mixtures like this.
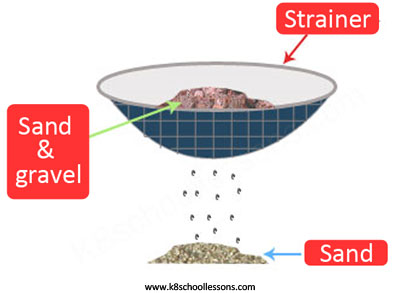
Example (2) – Water and sand mixture
Filtering is used to separate mixtures like water and insoluble substances such as sand.
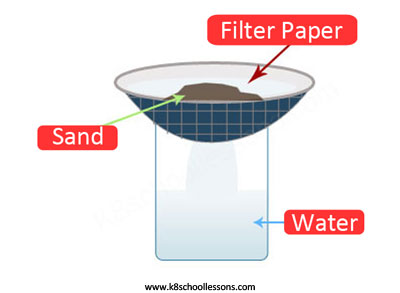
Example (3) – Sand and salt mixture
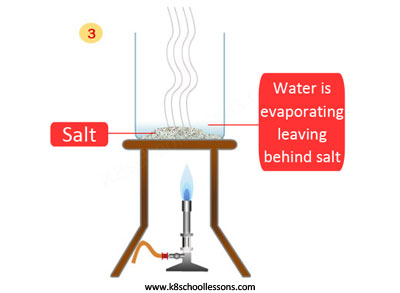
To separate a mixture of sand and salt, first the mixture should be mixed with water and then filtered, lastly evaporated.
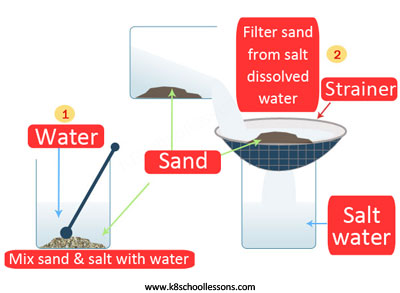
What is happening in these steps? First the salt dissolves in the water, then sand filters off and lastly, water evaporates to leave the salt.
Magnetism and crystallization are some advanced processes of physical changes.
The process of magnetism is reversible, and no new materials are formed as it does not affect the chemical composition.
The type, shape and the size of crystals can be changed by heating, rolling or hammering. However, no new materials are formed.
Also, read the lesson ‘Irreversible Changes‘ and try Reversible Changes and Irreversible Changes quizzes and worksheets.